


|

| 产地 | 中国 |
| 品牌 | Chemstan |
| 货号 | LC-01 |
| 用途 | 中间体 |
| 包装规格 | 20mg |
| 纯度 | 98%% |
| CAS编号 | 336113-53-2 |
| 是否进口 | 否 |
联合知名大学科研院所及企业开发药食两用植物标准品和天然植物有效单体,主打中药对照品/标准品/天然植物有效单体,小分子化合物库,药物杂质。所有产品仅用作科学研究,我们不为任何个人用途提供产品和服务。
Fields of Application :
Ispinesib (SB 715992) is a potent mitotic kinesin KSP (Eg5) inhibitor with a Ki and an IC50 of 0.6 and 4.1 nM, respectively.
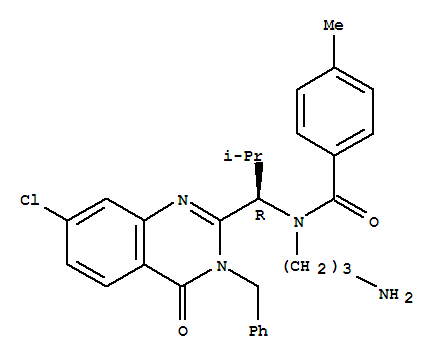
| CAS Number: | 336113-53-2 |
| Purity: | >98% |
| Molecular Weight: | 517.06 |
| Molecular Formula: | C30H33ClN4O2 |
Quality Control: HPLC、NMR、 LC/MS(Please contact us to get the QC report)
| Synonyms: | Ispinesib;SB-715992;SB715992 |
| Chemical Name: | |
| Storage: | 2 years -20°C Powder, 2 weeks4°C in DMSO,6 months-80°C in DMSO |
Note: Products for research use only, not for human use
Description:
Ispinesib (SB 715992) is a potent mitotic kinesin KSP (Eg5) inhibitor with a Ki and an IC50 of 0.6 and 4.1 nM, respectively. Ispinesib (SB 715992) has cytotoxic activity at less than 10 nM in a spectrum of tumor cell lines. Ispinesib (SB 715992) is negative in a mouse model of peripheral neuropathy in which paclitaxel is positive. Ispinesib (SB 715992) was shown to have activity against advanced human colon tumor xenografts including: Colo205 (complete regressi), Colo201 (complete regressi), and HT-29 (tumor growth delay). Ispinesib (SB 715992) (10 mg/kg) induced unexplained toxicity in mice bearing osteosarcoma xenografts. Ispinesib (SB 715992) enhanced the antitumor activity of trastuzumab, lapatinib, doxorubicin, and capecitabine and exhibited activity comparable with paclitaxel and ixabepilone.For the detailed information of SB-71599 (Ispinesib29), the solubility of SB-71599 (Ispinesib29) in water, the solubility of SB-71599 (Ispinesib29) in DMSO, the solubility of SB-71599 (Ispinesib29) in PBS buffer, the animal experiment (test) of SB-71599 (Ispinesib29), the cell expriment (test) of SB-71599 (Ispinesib29), the in vivo, in vitro and clinical trial test of SB-71599 (Ispinesib29), the EC50, IC50,and Affinity of SB-71599 (Ispinesib29).
References:
C(N(CCCN)[C@H](C1N(CC2=CC=CC=C2)C(=O)C2=C(N=1)C=C(Cl)C=C2)C(C)C)(=O)C1=CC=C(C)C=C1